Our main (and everchanging) research goals with non-exhaustive list of papers published are:
- Determine the factors that influence generation, maintenance and function of memory CD8 T cells; Nat Immunol 3, 619-626 (2002) / Nat Med 11, 748-756 (2005) / PLoS Pathogens 11, e1005219 (2015)/ Cell Rep 31: 107508 (2020) / Nat Immunol 23: 386-398 (2022)/ Immunity 57:1878-1892 (2024)
- Characterize CD8 T cells responding to multiple rounds of antigenic stimulations; Immunity 33, 128-140 (2010) / J Immunol 192, 5652-5659 (2014) / Cell Rep 24: 3374-3382 (2018) / CE J Immunol 204: 1431-1435 (2020) / eLife 10: e68662 (2021) / J Immunol 210: 168-179 (2023) / Cell Rep 44: 115247 (2025)
- Establish relevant experimental models to study antigen-experienced CD8 T cells in vivo; Immunity 26, 827-841 (2007) / J Immunol 183, 7672-7681 (2009) / Front Immunol 8: 1527 (2017) / J Clin Invest 129: 3894-3908 (2019) / Immunity 52: 419-421 (2020) / Immunohorizons 6: 528-542 (2022) / J Immunol 214: 995-1007 (2025)/ Nat Immunol 26: 1087-1098 (2025)
- Investigate the alterations in T cell-mediated immunity that occur after sepsis; J Immunol 192, 3618-3625 (2014) / PLoS Pathog 13: e1006569 (2017) / J Immunol 200: 1543-1553 (2018) / eLife 9: e55800 (2020) / J Immunol 207: 1871-1881 (2021) / eLife 10: e70989 (2021) / J Immunol 210: 168-179 (2023)/ Front Immunol 14: 1130009 (2023) / PLoS Pathog 10: e1011720 (2023) / J Immunol 212: 563-575 (2024)
- Define sepsis-induced state of immunoparalysis and explore treatments to restore immunity in sepsis survivors; J Immunol 197: 4301-4311 (2016) / PLoS Pathog 14: e1007405 (2018) / J Immunol 202: 2843-2848 (2019) / J Immunol 206: 1171-1180 (2021) / Immunohorizons 5: 477-488 (2021) / J Immunol 221: 711-719 (2023) / JCI Insight 9: e175785 (2024) / Shock 63: 189-201 (2025)
- Characterize recovery, maintenance, and function of naive and memory T cell subsets following radiation exposure; PNAS 120: e2302785120 (2023)/ J Exp Med 221: e20231144 (2024) / JCI Insight in press (2025) / J Immunol in press (2025)
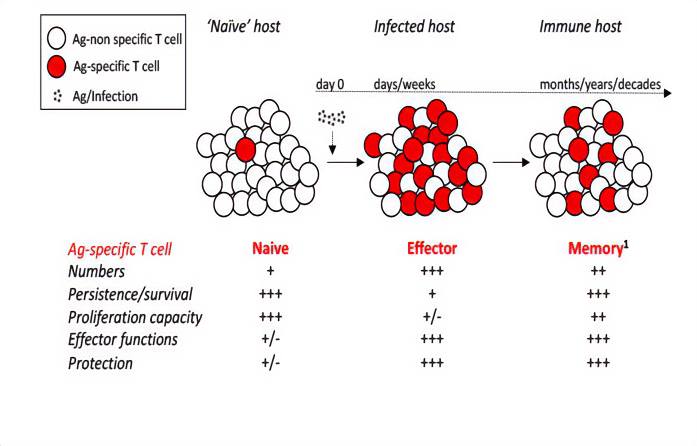
Naive, effector, and memory T cells generated after acute infection/vaccination. Naïve T cells exist at low numbers with minimal functionality and protective capacity but vigorously proliferate upon cognate Ag-stimulation, generating a sizable effector pool with ample functionality and protective capacity but have a diminished life span. The effector T cells that survive the contraction phase will form a heterogeneous population of long-lived memory
CD8 T cells and maintain their effector function and protective capability.
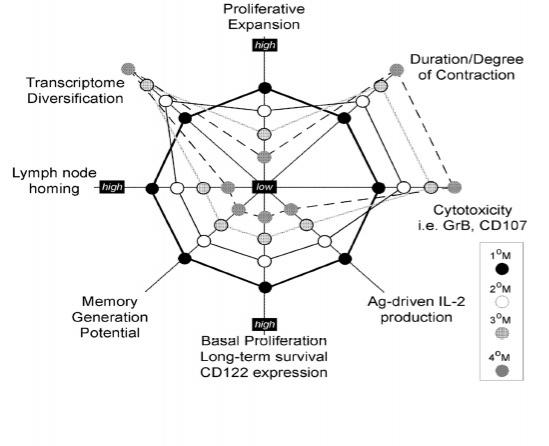
Memory CD8 T cell phenotype and function is dependent on the history of Ag-encounters.Functional characteristics of memory CD8 T cells that have encountered Ag multiple (1-4) times. Functional abilities and characteristics are highest for memory populations at the outermost edges and progressively decrease for memory populations moving inward (i.e. proliferative expansion 1°>2°>3°>4°)
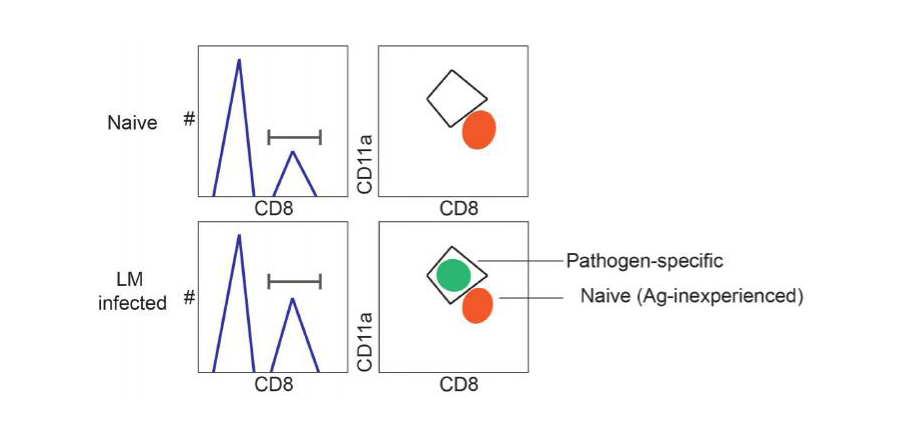
Pathogen-specific CD8 T cells can be identified using 'surrogate activation markers' approach.
After infection, pathogen-specific CD8 T cells upregulate the surface expression of the integrin CD11a and downregulate surface expression of CD8a, while surface expression of these molecules remains unchanged in naïve Ag-inexperienced CD8 T cells.
These surface phenotype changes can help distinguish pathogen-specific from naïve CD8 T cells after infection without knowledge of specific antigenic epitopes or host MHC restriction elements.
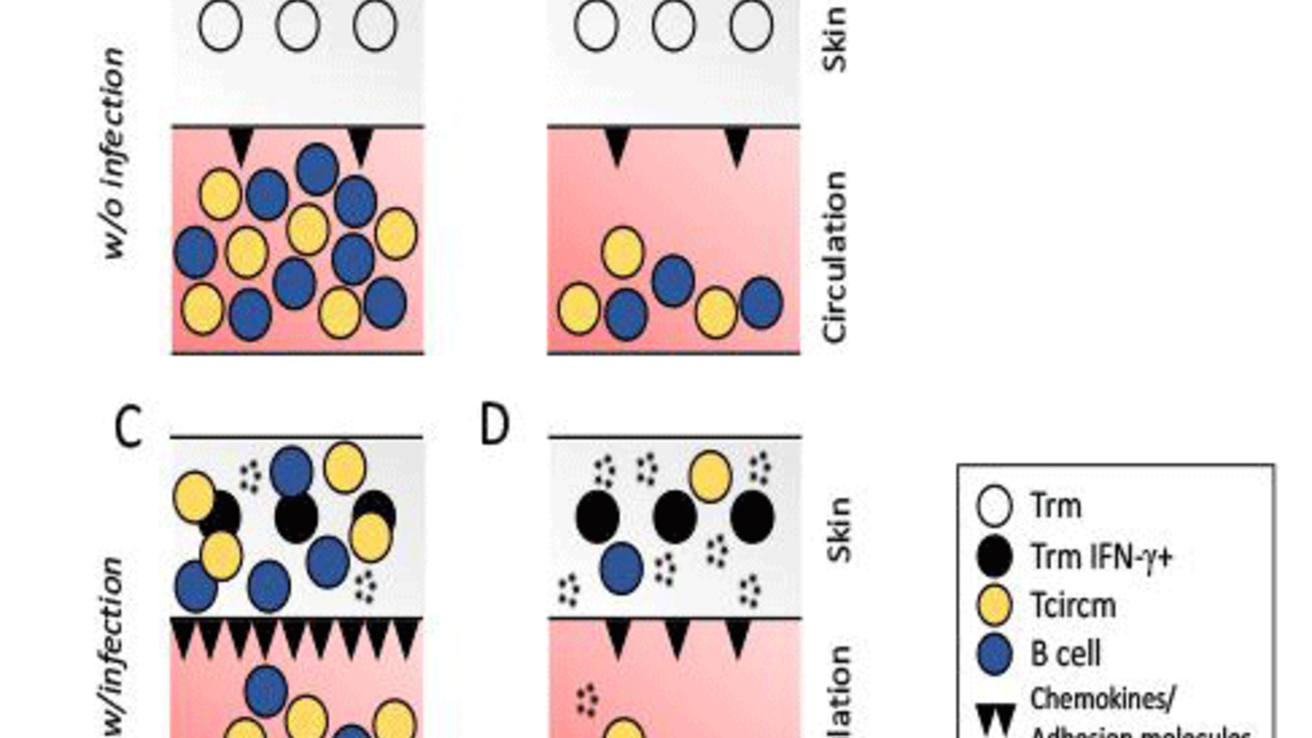
Sepsis diminishes localized CD8 T cell-mediated immunity. In a healthy host, tissue resident memory CD8 T cells (TRM) and circulating memory (TCIRCM) CD8
Tand B cells are evoked upon infection (A) and re-infection induces TRM production of IFN-γ, endothelial cell upregulation of chemokines and adhesion molecules, influx of memory T and B cells from the circulation and pathogen clearance (C). Sepsis induces a dramatic numerical loss of TCIRCM but not TRM (B), endothelial cells are unable to respond to the IN-γ signal, few effector cells are recruited from the circulation and pathogen clearance is significantly impaired (D).